Regulatory Services
Bringing a new pet drug to market requires meeting complex regulatory requirements. Regulatory affairs is not merely about submitting documents; it is a strategic task that spans the entire product lifecycle, aimed at ensuring that every pet drug launched not only treats diseases but also maximizes the safety of medication use for pets.
While the regulatory process for pet pharmaceuticals is complex, it is not a barrier. Protheragen PawDiscover is a reliable partner in pet drug regulatory, offering clients comprehensive regulatory services. We possess a team with deep experience and exceptional expertise in veterinary regulatory. Not only are we well-versed in the regulations of the US FDA-CVM, EU EMA-CVMP, and other national regulatory bodies, but we also understand the unique requirements of pet drug development (such as differences between dog breeds and cat breeds, palatability considerations, etc.). We can efficiently navigate the entire process, helping our clients shorten the time-to-market for their pet drugs. Please do not hesitate to contact us to discuss how our regulatory services can accelerate your pet pharmaceutical's journey to market.

Our Services
Our regulatory services are designed to provide comprehensive support throughout the entire lifecycle of your pet drug. This encompasses all aspects, including drug development strategy, risk management, clinical affairs, medical affairs, and post-marketing surveillance. Our goal is to minimize delays, reduce risks, and ensure your pet drug meets the highest standards of safety, efficacy, and quality required by global regulatory authorities. Whether you are a startup biotech company or an established pharmaceutical enterprise, we tailor our services to your specific needs and stage of development.
Regulatory Strategy & Intelligence
- Regulatory Strategy
- Regulatory Gap Analysis
- Assist with Regulatory Authority Meetings
Regulatory Submissions & Documentation
- Investigational Pet Drug Submissions
- Pet Drug Marketing Authorization Application
- Label Preparation and Review
- Writing Various Medical Documents
Regulatory Operations & Compliance
- eCTD Publishing & Submission Management
- Regulatory Information Management
- Labeling Development & Compliance
- GMP/GCP/GLP Compliance Support
Lifecycle Management & Post-Marketing Support
- Advertising and Promotion Material Review
- Change Control Strategy & Submissions
- Product Defect & Recall Support
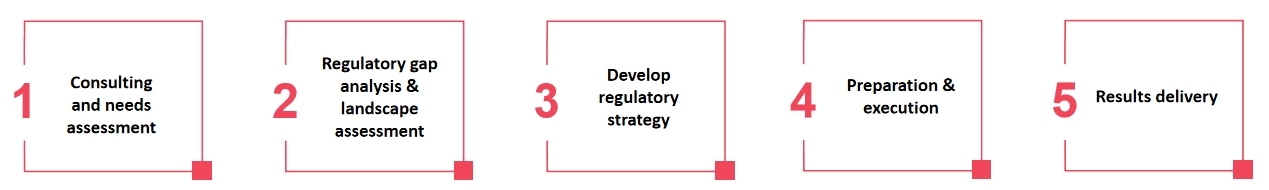
Process of Our Services
Why Choose Us?
End-to-End Lifecycle Support
We provide comprehensive services, spanning from early development and strategic planning to post-market compliance and lifecycle management, ensuring seamless support at every stage.
Proactive and Strategic Approach
We don't just react; we anticipate. Our focus is on early strategic planning, proactive agency engagement (pre-submission meetings), and risk mitigation, preventing costly surprises and de-risking your development pathway.
Proven Efficiency & Quality
We possess an intimate understanding of the nuances of global regulatory, and continuously optimize regulatory processes to faster turnaround times for document preparation, review cycles, and query responses. We maintain the highest standards of quality and compliance in every submission.
Global Reach with Local Insight
We develop strategies for global markets and possess the network and expertise to navigate regional requirements effectively. We manage the complexities of multi-country submissions.



