Clinical Trial Services
Bringing a new pet drug to market requires rigorous scientific validation, and clinical trials are the indispensable bridge from laboratory discovery to safe, effective, and commercially available medication. Protheragen PawDiscover, a company specializing in pet drug research and development, offers comprehensive pet drug clinical trial services. Our expert team possesses an in-depth understanding of the regulatory requirements for pet drugs and can effectively navigate the challenges inherent in pet drug clinical trials.
Whether you are an emerging biotech company or an established pharmaceutical firm, we can tailor clinical trial protocols to meet the unique needs of your pet drug, taking into account its characteristics, target indications, and development stage, ensuring compliance with regulatory standards. If you are seeking a reliable clinical trial partner, please do not hesitate to contact us. We are committed to helping you expedite the market launch of your pet drug.
Our Services
Pet drug clinical trials refer to systematic studies conducted on pets to investigate the effects and adverse reactions of investigational drugs, as well as their absorption, distribution, metabolism, and excretion patterns. The purpose is to determine the efficacy and safety of the investigational drugs, primarily including Phase I-IV clinical trials.
Protheragen PawDiscover offers one-stop pet drug clinical trial services that encompass every stage, from initial feasibility assessment and protocol design to patient recruitment, trial execution, data analysis, and regulatory submission support. Our clinical trials encompass a range of pet health areas, including oncology, cardiovascular diseases, dermatology diseases, neurology diseases, infectious diseases, and other diseases.
- Feasibility Analysis
- Clinical Trial Protocol
- Investigator's Brochure Development
- Patient Recruitment and Retention
- Phase I Clinical Trial
- Phase II Clinical Trial
- Phase III Clinical Trial
- Phase IV Clinical Trial
- Clinical Monitoring
- Data Management
- Biostatistical Analysis
- Adverse Event (AE) / Adverse Drug Reaction (ADR) Collection & Reporting
- Drug Safety and Pharmacovigilance
- Clinical Study Report Authoring
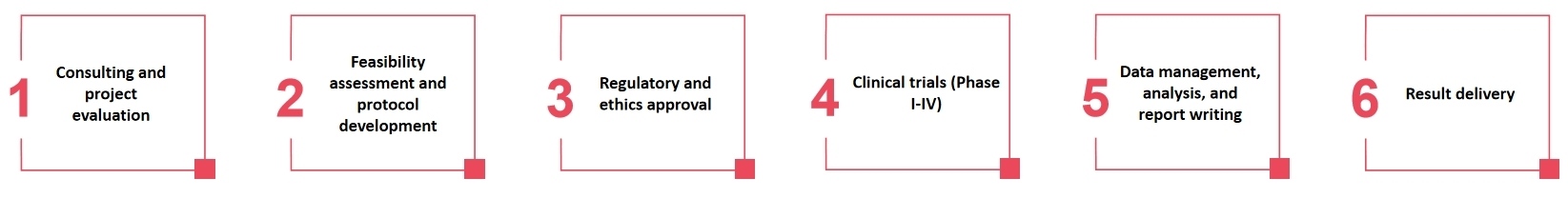
Process of Our Services
Why Choose Us?
Expert Team
Our team is not only well-versed in clinical trial protocols but also possesses a deep understanding of the species-specific physiology, pathology, and behavior of pets (dogs and cats). This ensures that trial designs are more rational, data interpretation is more accurate, deviations are minimized, and accelerated clinical trial initiation.
Regulatory Compliance Excellence
Our trials strictly adhere to Good Clinical Practice (GCP), veterinary regulatory guidelines, and our own rigorous Standard Operating Procedures. This ensures efficient submission of application materials, timely communication with regulatory authorities, and effective project advancement, significantly shortening the pet drug clinical trial cycle.
Excellence in Data Management
With extensive experience in clinical data management, we utilize advanced data cleaning and statistical analysis tools to deliver clear, compelling, and defensible data. This provides the strongest possible evidence for the efficacy of your pet drug.
Flexible and Efficient Customized Services
We understand that each project is unique. Rejecting a one-size-fits-all approach, we provide flexible and tailored services based on your drug's characteristics, target indications, development stage, and specific budget.



